Abstract
Nevus comedonicus (NC) was initially described by Kofmann in1895 and scripted as a “comedo nevus”. Nevus comedonicus is cogitated as an exceptional subtype of epidermal nevus engendered from the hair follicle and a lesion predisposition for face and neck. Nevus comedonicus syndrome with constituent nevus comedonicus was initially coined by Engber in 1978. Delayed milestones and deferred neonatal development can ensue with nevus comedonicus or nevus comedonicus syndrome1, 2.
Author Contributions
Academic Editor: Carneiro S, Federal University of Rio de Janeiro, Brazil.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2020 Anubha Bajaj
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Preface
Nevus comedonicus (NC) was initially described by Kofmann in1895 and scripted as a “comedo nevus”. Nevus comedonicus is cogitated as an exceptional subtype of epidermal nevus engendered from the hair follicle and a lesion predisposition for face and neck. Nevus comedonicus syndrome with constituent nevus comedonicus was initially coined by Engber in 1978. Delayed milestones and deferred neonatal development can ensue with nevus comedonicus or nevus comedonicus syndrome1, 2.
Disease Characteristics
Nevus comedonicus is a condition of obscure aetiology, a category of epidermal nevus or an origin as a hamartoma primarily derived from mesodermal segments of the pilosebaceous unit. Genetic mosaicism can be proposed as an aetiological factor for emergent nevus comediconus 1.
Estimated prevalence of nevus comedonicus varies from 1 : 45000 to 1 : 100,000 individuals. The condition is devoid of a specific gender or racial predilection and male to female proportion of disease emergence is equivalent. Nevus comedonicus can emerge as a congenital condition or within the neonatal period in an estimated 50% subjects and majority of instances are discerned prior to 10 years1, 2.
Disease Pathogenesis
Chromosomal mutation of fibroblastic growth factor receptor 2 (FGFR2) along with enhanced enunciation of interleukin 1- alpha are cogent factors within the pathogenesis of nevus comedonicus. Gamma- secretase and filaggrin are also incriminated. Inter-relation of fibroblastic growth factor (FGF) and fibroblastic growth factor receptor 2 (FGFR2) is a critical component of mesenchymal- epithelial interaction, particularly in the genesis of pilo-sebaceous unit. FGFR is composed of a family of tyrosine kinase receptors wherein FGFR2b is singularly situated within the epithelial cells, especially epidermal keratinocytes and sebocytes. Activation of FGFR2 signalling pathway in association with enhanced manifestation of interleukin 1- alpha is delineated with the occurrence of nevus comedonicus2, 3. Somatic mutations, as cogitated with Ser252Trp substation, can be exhibited in individuals with segmental acne. Gamma- secretase, detected within hair follicular epithelium, is also implicated in the origin of nevus comedonicus, as the absence of cogent enzymes ensures a comprehensive conversion of hair follicle into epidermal cysts.
Somatic mutations within NEK9 are implicated in the genesis of nevus comedonicus, a gene which significantly regulates follicular homeostasis. Genomic mutations of NEK9 are accompanied by an enhanced phosphorylation at Thr 210 position, thereby indicating the activation of NEK9 associated kinase2, 3.
Configuration of comedones in nevus comedonicus are associated with adjunctive modifications such as decimated markers of follicular differentiation and ectopic enunciation of keratin 10 besides an upregulation of ABCA123.
Clinical Elucidation
Nevus comedonicus is frequently asymptomatic and usually represents grouped, distended, follicular apertures delineating dark-staining keratin plugs, commonly situated upon the face, neck, upper arms, chest and abdomen whereas scalp, palms, soles and genital region as with glans penis are infrequently incriminated. Generally, a singular group of dilated, closely arranged, adjacent and plugged follicular ostia are exemplified within a honey comb pattern. Nevus comedonicus typically enunciates dilated, follicular apertures plugged with keratinous material which recapitulate classical comedones3, 4.
Aggregated, dilated, plugged follicular ostia predominantly configure a honey comb pattern. Plugged ostia are impacted with lamellated, keratinaceous substance, the superficial surface of which simulates black dots, wherein keratinous substance is resistant to mechanical extraction, in contrast to acne comedones3, 4. Nevus comedonicus is variously distributed as unilateral, bilateral, linear, interrupted, segmental and a Blaschkoid pattern. Lesion distribution as linear, segmental or Blaschkoid is common whereas bilateral lesions are exceptional.
Nevus comedonicus delineates specific subtypes such as a) a non pyogenic form with acne-like characteristics, additionally delineated as Munro’s acne nevus, which can be considered as a condition distinct from nevus comedonicus4, 5.
b) a category demonstrating the articulation of cysts, pustules, papules and abscesses in diverse stages of configuration.
Nevus comedonicus can occur as a solitary lesion or in concurrence with systemic anomalies of the central nervous system, skeletal system, ocular or dental aberrations and associated cutaneous manifestations. Diverse combinations of aforesaid clinical manifestations configure the nevus comedonicus syndrome4, 5. Nevus comedonicus syndrome is a component of epidermal nevus syndrome with cogent extra-cutaneous manifestations in association with disorders like nevus comedonicus, verrucous epidermal nevus and nevus sebaceous.
Nevus comedonicus syndrome is defined as a neuro-cutaneous disorder with nevus comedonicus occurring in association with extra-cutaneous manifestations such as skeletal, ocular, tooth and central nervous system anomalies. Frequently encountered are features such as cataracts, scoliosis, fused vertebrae, spina bifida and delayed mental development. Additionally, seizures, paresis, dysgenesis of corpus callosum, aberrant electroencephalogram, microcephaly, transverse myelitis, limb deformities as with the absence of fifth finger, polysyndactyly or clinodactyly, syndactyly, supernumerary digits and oligodontia as a common dental aberration are exhibited in nevus comedonicus syndrome5, 6.
Nevus comedonicus syndrome as a constituent of epidermal nevus syndrome accompanies disorders such as Schimmelpenning syndrome, phacomatosis pigmentokeratotica, angora hair nevus syndrome and Becker nevus syndrome, in concurrence with adjunctive syndromic manifestations wherein a genetic component remains unestablished.
Exceptional, systemic syndromes associated with nevus comedonicus are constituted by Alagille’s syndrome, spinal dysraphism, hypothyroidism and Paget’s bone disease5, 6.
Sufficient mechanical stress is contemplated as a trigger for the occurrence of hidradenitis suppurativa in concurrence with nevus comedonicus.
Primary dermatological disorders accompanying nevus comedonicus are basal cell carcinoma, squamous cell carcinoma, keratoacanthoma, linear morphea, lichen striatus, accessory breast tissue, epidermolytic hyperkeratosis, nevoid hyperkeratosis of aerola, hidradenoma papilliferum, syringocystadenoma papilliferum, epidermoid cysts, cutaneous horns, haemangiomas, ichthyosis, Sturge- Weber syndrome, haemangiomas, inverse acne leukoderma, pilar sheath tumour, dilated pore of Winer, trichoepithelioma and adjunctive epidermal nevi.
Subjects with nevus comedonicus require appropriate evaluation for nevus comedonicus syndrome. Intermittent disease flares can be observed4, 6.
Histological Elucidation
A cogent tissue specimen demonstrates typical features of comedones as follicular ostia distended and impacted with keratin. Significant histological manifestations are the occurrence of enlarged, grouped, distended follicular ostia demonstrating an absence of hair shafts along with impaction of laminated keratin. Base of the follicular invaginations may or may not delineate singular, rudimentary glandular structures. Miniature cysts, cystic invaginations and occasional enlarged cysts can be encountered. Cystic articulations are diverse and preponderantly layered by keratinizing, stratified squamous epithelium. Hyperkeratosis or epidermolytic hyperkeratosis and acanthosis of superimposed epidermis can concur although the lesion is devoid of parakeratosis or dyskeratosis5, 6.
Immune histochemical evaluation depicts an enhanced expression of proliferating cell nuclear antigen (PCNA), intercellular adhesion molecule 1 (ICAM-1), histocompatibility antigens (HLA- DR) and CD68.
Nevus comedonicus demonstrates immune reactivity to cytokeratin, simulating the expression of normal cutaneous surfaces. Generally encountered within the granular epidermal layer, immune reactivity for filaggrin is demonstrable within the entire epidermal thickness of closed comedones, a molecular which can be implicated in the genesis of nevus comedonicus6, 7. Electron microscopy demonstrates an enhanced quantification of Langerhans cells, numerous kerato-hyaline granules along with an abundance of tonofilaments within upper portion of stratum spinosum. Incompletely differentiated arrector pili muscles are impacted with intracellular glycogen particles6, 7. Figure 1, Figure 2, Figure 3, Figure 4, Figure 5, Figure 6, Figure 7, Figure 8, Figure 9, Figure 10.
Figure 1.Nevus comedonicus with grouped, dilated follicular ostia, impacted with keratin and a layering of hyperkeratotic stratified squamous epithelium11.
Figure 2.Nevus comedonicus with plugging of follicular ostia, keratinous impaction and a lining of hyperkeratotic stratified squamous epithelium11.
Figure 3.Nevus comedonicus with aggregated follicular ostia, lamellated keratin, hyperkeratotic stratified squamous epithelial lining and an attenuated superimposed epithelium12.
Figure 4.Nevus comedonicus with distended follicular ostia, keratin accumulation, hyperkeratotic epithelial lining and intervening loose fibro-connective tissue13.
Figure 5.Nevus comedonicus clinically demonstrating darkly-stained, grouped, distended, follicular ostia, plugged with keratin, demonstrating a honey-comb pattern14.
Figure 6.Nevus comedonicus delineating dilated, plugged, follicular ostia, keratinous substance and a layering with a hyperkeratotic, stratified squamous epithelium[15.
Figure 7.Nevus comedonicus with an aggregation of dilated, plugged follicular ostia, keratinous flakes and an intensely hyperkeratotic stratified squamous epithelial layer16.
Figure 8.Nevus comedonicus exhibiting aggregates of distended follicular ostia, keratin plugging and a lining of hyperkeratotic stratified squamous epithelium16.
Figure 9.Nevus comedonicus exemplifying a dilated, plugged follicular ostium, keratinous flakes and a layer of stratified squamous epithelium17.
Figure 10.Nevus comedonicus depicting an impacted, distended follicular ostium, keratinous aggregation and a superimposed, acanthotic, hyperkeratotic, stratified squamous epithelium17.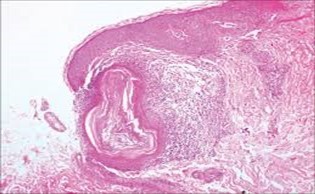
Differential Diagnosis
Atypical representations of nevus comedonicus require a demarcation from adjunctive conditions such as atypical acne, segmental acne or associated mosaic acneiform disorders such as chloracne and Favre- Racouchot syndrome, denominating nodular elastosis with cysts and comedones. Disorders such as familial dyskeratotic comedones mandate a segregation from nevus comedonicus and are cogitated as exceptional, autosomal dominant, inherited conditions with characteristic emergence of hyperkeratotic comedonal lesions. Histological assay demonstrates the occurrence of comedones with associated dyskeratosis6, 7.
Investigative Assay
Generally, a clinical discernment of nevus comedonicus is adequate on account of preliminary age of disease onset and a typical morphology. Systematic evaluation of central nervous system, skeletal system and ocular structures are necessitated to exclude nevus comedonicus syndrome7.
Employment of a contemporary diagnostic modality such as dermoscopy is beneficial in determining nevus comedonicus. Typical comedonal lesions are highlighted by dermoscopy which characteristically demonstrates multiple, light and dark brown, spherical and barrel shaped, homogenous zones embedded with prominent keratin plugs8.
Therapeutic Options
Spontaneous resolution of nevus comedonicus is unknown. Frequently, the superimposed epithelium is hyperkeratotic and occasionally acanthotic, thus extraction of a comedo is difficult, in contrast to removal of acne comedones.
The essentially benign nevus comedonicus may not mandate the adoption of aggressive treatment modalities, except in complex instances or for aesthetically pleasing outcomes8, 9.
Treatment is primarily adopted for cosmetic reasons or for alleviation of complications such as configuration of cysts or abscesses. Cosmetic management for post- inflammatory scarring may be necessitated.
Topical and systemic retinoids can be adopted singularly or in combination with topical steroids for inciting anti-inflammatory functions.
A pore strip can be successfully employed to treat keratin plugs8, 9.
Conservative therapeutic options comprise of application of emollients and moisturizers, topical corticosteroids in inflammatory lesions and keratolytics as salicylic acid or 12% ammonium lactate solution. Conservative treatment modalities can enhance cosmetic outcomes in certain subjects. As retinoids are efficacious in treating acne, the agents can be administered in nevus comedonicus. Application of topical tretinoin produces limited outcomes. Application of topical tazarotene as monotherapy or in combination with topical mometasone furoate or calcipotreine can produce superior outcomes in nevus comedonicus9. However, lesion resolution is incomplete and relapses are frequent with topical applications. Oral retinoids such as isotretinoin are ineffective in majority of instances although are beneficial in disseminated, systemic inflammatory variants. Topical adapalene and systemic or topical bexarotene can be administered, although the outcomes remain undetermined9, 10.
Administration of FGFR inhibitors with cogent anti-vascular activity can be considered. Contemplated contemporary treatment modalities are comprised of administration of fibroblastic growth factor receptor (FGFR) inhibitors, interleukin 1- alpha inhibitors and anti gamma -secretase agents.
Interleukin 1- alpha inhibitors are classified into receptor inhibitors such as anakinra and monoclonal antibodies against the interleukin molecule and can be adopted for treating nevus comedonicus, although the outcomes are insufficiently detailed9, 10.
Administration of gamma- secretase, especially stimulators of aforesaid enzymes such as general control nonderepressible 2 (GCN2), a subunit of eukaryotic translation factor 2 kinase, can be contemplated as a future therapeutic option10.
Surgical extermination of lesions of nevus comedonicus can be advantageous. Focal lesions of nevus comedonicus can be extracted with a cogent surgical eradication and demonstrates superior aesthetic outcomes, in contrast to adoption of manoeuvers such as superficial shaving, comedo extraction and techniques of dermabrasion.
Surgical eradication of linear nevus comedonicus situated upon the limbs mandate a concomitant, complex decongestive therapy, especially manual lymphatic drainage and employment of compression garments, wherein aforesaid measures enhances wound healing. Enlarged lesions require cutaneous and soft tissue transplantation for closure of surgical defects. Alternatively, repetitively-mobilizing or self-mobilizing osmotic tissue expanders can be utilized to obtain surplus skin for closure of discontinuous wounds9, 10. Lasers are efficaciously adopted in the treatment of nevus comedonicus. Application of ablative lasers is beneficial. Ultra-pulse carbon dioxide laser and erbium- YAG laser can be adequately utilized. 1450- nm diode laser can be gainfully employed in treating nevus comedonicus. Also, laser induced stimulation of sub-lesional collagen can decimate epidermal invagination with consequent enhancement of skin texture. Laser therapy with the configuration of 2940- nm erbium YAG, 10,600-nm ultra-pulsed carbon dioxide laser or 1450-nm diode laser demonstrate beneficial results in specific candidates. Crater or depression of cutaneous surfaces remain10.
Erbium YAG laser therapy is frequently followed by delayed relapse of the lesion. In contrast to application of ablative lasers, 1450-nm diode laser can shrivel sebaceous glands and diminish seborrhoea. Combined 1450-nm diode laser and 1550-nm erbium- doped fibre laser can be employed to treat nevus comedonicus10. Table 1.
Table 1. Differential Diagnosis of Nevus Comedonicus 2| Nevus Comedonicus | Tricho-folliculoma | Dilated port of Winer | Pilar sheath acanthoma | Folliculo-cystic and collagen comedo hamartoma | Hair Cortex | |
| Site | Head | - | - | - | Head | Head |
| Face | Face | Face | Face | - | ||
| Neck | - | - | - | - | Neck | |
| Trunk | Trunk | - | - | Trunk | Trunk | |
| Limbs | - | - | - | - | - | |
| Follicles | Numerous open follicles | Open “mother” follicles | Open “mother” follicles | - | Numerous open follicles | Solitary lesion |
| Cysts | Present | Present | Present | |||
| Hair shafts | - | Vellus shafts | - | - | - | - |
| Lamellated keratin | Present | - | - | - | - | |
| Others | - | - | - | Massive epithelial proliferations without follicles | Abundant collagen formation | - |
References
- 1.Kofmann S. (1895) Ein Fall von seltener Lokalisationa und Verbreitung von Komedonen” Arch Dermatol Syphilol. , (Berlin) 32-177.
- 2.Tchernev G, Ananiev J. (2013) Nevus comedonicus: an updated review” Dermatology and Therapy. 3, 33-40.
- 3.Kaliyadan F, Karalikkattil T A. (2020) Nevus Comedonicus Stat Pearls Publishing:Treasure Island. , Florida
- 5.Happle R. (2010) The group of epidermal nevus syndromes Part I Well defined phenotypes“. , J Am Acad Dermatol 63, 1-22.
- 6.Jeong H S, Lee H K. (2012) Multiple large cysts arising from nevus comedonicus” Arch Plast Surg. 39, 63-6.
- 7.Zarkik S, Bouhllab J. (2012) Keratoacanthoma arising in nevus comedonicus”. , Dermatol Online J 18, 4.
- 8.Liu F, Yang Y. (2018) Mutation and expression of ABCA12 in keratosis pilaris and nevus comedonicus” Mol Med Rep. 18(3), 3153-3158.
- 9.Zanniello R, Pilloni L. (2019) Late-onset nevus comedonicus with follicular epidermolytic hyperkeratosis- case report and review of literature”. , Am J Dermatopathol 41(6), 453-455.