Abstract
Introduction:
Data support the use of both ultrasound (US) and magnetic resonance imaging (MRI) in the prenatal prognostication of congenital diaphragmatic hernia (CDH). The aim of this study was to examine our experience and learning curve with both of these diagnostic tools in the setting of a new fetal program.
Materials and Methods:
This is a case series performed as a quality improvement measure. Fetuses were identified at a single tertiary institution with both ultrasound lung-to-head ratio (LHR) and MRI fetal lung volume from December 2012 until July 2016. Prenatal and postnatal data were collected. Statistical analysis was performed and a p-value of <0.05 was considered significant.
Results:
Twenty-one patients met inclusion criteria. Inaccurate LHRs were found in 26.9% (7/26) of patients, with the lack of a four-chamber heart view as the most common inaccuracy (5/26, 19.2%). Patients with only some or no stomach in the thoracic cavity on fetal MRI had 100% survival to discharge.
Discussion:
Accurate prenatal prognostication of CDH is challenging. We identified a pitfall in attaining LHR that can be easily identified, and that may influence the accuracy of the measurement. Furthermore, stomach position on MRI is a relatively newly described quick, easy, and reproducible metric for predicting prognosis.
Author Contributions
Academic Editor: Ahmed el-sabbagh, Faculty of medicine,Mansoura university, Egypt.
Checked for plagiarism: Yes
Review by: Single-blind
Copyright © 2017 Shannon M Koehler, et al.
 This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Competing interests
The authors have declared that no competing interests exist.
Citation:
Introduction
Congenital diaphragmatic hernia (CDH) occurs in 1 out of 2200-4000 live births1, 2, 3. The overall postnatal mortality rate varies widely and has been reported from 9.3-79%3 in the literature. The mortality rate is partly due to associated anomalies, as 20-40% of CDH patients will have a major malformation 3. In patients with isolated CDH, the mortality rate is attributable to severe pulmonary hypoplasia and pulmonary hypertension, neither of which have viable postnatal treatment options. Extracorporeal membrane oxygenation (ECMO) can be utilized for patients with severe pulmonary hypertension, but comes with the risk of neurologic injury and bleeding4, 5. Likewise, inhaled nitric oxide (iNO) has been utilized to treat pulmonary hypertension but, like ECMO, there have been randomized control trials and Cochrane reviews which have failed to show significant benefit6, 7, 8, 9. The use of sildenafil has also been described in the management of pulmonary hypertension; however, a randomized control trial has not been performed to verify the efficacy of this treatment. This leaves the practitioner with the current tenants of CDH management, namely permissive hypercapnia and avoidance of high inspiratory pressures2. Patient care by a multidisciplinary team at a tertiary, high volume center with institutional guidelines that include the aforementioned tenants, has led to improved survival10, 11. While roughly 80% of CDH cases are now prenatally diagnosed12, 13, predicting which neonates may not survive or may have poor outcomes can be enigmatic. More accurate prenatal prognostication for these fetuses may allows families to make better informed decisions regarding the management of their pregnancy, the aggressiveness of fetal or postnatal interventions, and help set family expectations.
Currently, numerous prenatal imaging modalities and measurements are utilized to predict survival in CDH. Ultrasound measured lung-to-head ratio (U/S LHR) is commonly used, but has several pitfalls including the following: different methods of calculating lung area can alter the final measurement14, 15, use of ultrasound is operator-dependent, mastering the technique requires a steep learning curve, and the predictive value can vary by gestational age at which it was obtained14, 16. MRI measured fetal lung volumes (MRI FLV) are also often obtained at fetal centers, but it too has several downfalls including: special software requirement and a highly trained radiologist to obtain the measurements, measurements are time-consuming, and these measurements require a comparative normogram such that the impact of normal lung development by gestational age is lessened. The ideal imaging modality would be straight-forward, rapidly obtained, and reproducible by a variety of users.
This is a description of the prenatal imaging utilized to counsel families with prenatal diagnosis of CDH at one institution during the inception of its fetal treatment program. In our case series we compare the use of U/S LHR and MRI FLV to survival in this patient population. We also discuss methods used to improve our performance and interpretation of prenatal imaging, ensuring optimization of the calculation of these prognostic data points, to help other similar centers.
Materials and Methods
This study was performed as a quality improvement measure at a single tertiary center along with the commencement of its fetal center. The IRB deemed this project “not human subject research” and required no further review or informed consent for data use. All fetuses with prenatally diagnosed CDH that underwent at least one prenatal ultrasound with LHR calculation, and at least one fetal MRI with FLV calculation, were identified in the radiology archives. The first image collected was in September of 2012 and the last image collected was in July 2016.
Accuracy of U/S LHR
For each patient, the original ultrasound wherein the LHR had been calculated was reviewed, and the U/S LHR noted. A single reviewer (EP) analyzed these ultrasound images to determine the accuracy of of U/S LHR calculation. All practitioners in the Maternal Fetal Medicine department use the same technique for calculating LHR, the longest diameter method15. The images were reviewed to ensure that the cross-sectional area of the contralateral lung was measured at the four chamber view of the heart (Figure 1), the cross-sectional area was calculated with the longest diameter in the correct plane, the width was perpendicular in the correct plane, and the actual calculation was mathematically accurate. The U/S LHR values were then calculated by entering these measurements into the LHR calculator on perinatology.com. The inaccurate calculations were categorized based on the reason for inaccuracy: not measured at the level of the four chamber view of the heart, inaccurate calculation, poor images, no calipers, or oblique angle.
Figure 1.Fetal ultrasound demonstrating apropriate measurement of lung at the four-chamber view of the heart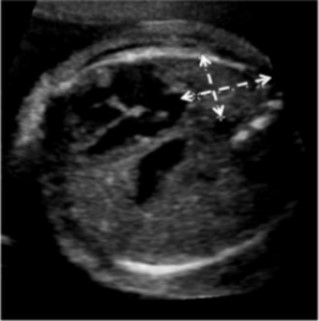
Predictors of Outcome
Charts were retrospectively reviewed for observed-to-expected (O/E) values for U/S LHR, MRI FLV measurements, and outcome of the pregnancy and fetus. O/E values normalize measurements to the gestational age of the fetus. MRIs were also retrospectively reviewed by a single pediatric radiologist for location of the stomach. Stomach location was simply defined as all, some, or none of the stomach in the thorax (Figure 2). The primary outcome was survival to discharge. Ultrasound O/E LHR and MRI O/E FLV was compared for all fetuses. An U/S LHR < 1, U/S O/E LHR < 15%, and MRI O/E FLV <25% were considered predictors of poor outcome, and these values were utilized to determine if the various measurements correlated.
Figure 2.MRI demonstrating the entire fetal stomach (black arrow) in the thoracic cavity. The white arrow points to the fetal diaphragm.
Statistics
Fisher exact probability tests and student’s t-tests were utilized to analyze the data where appropriate. P-value < 0.05 was considered significant.
Results
A total of 21 patients were identified with a prenatal diagnosis of CDH who had undergone both prenatal ultrasounds with LHR calculation, and a prenatal MRI with FLV calculation (Figure 3). Two of these patients were included in the accuracy of U/S LHR section, but excluded in the remaining analysis as they had either a right sided or bilateral CDH.
Figure 3.Flowchart showing outcomes of prenatally diagnosed CDH patients with both prenatal U/S LHR and MRI FLV obtained at Children’s Hospital of Wisconsin.
The remaining 19 patients that met inclusion criteria had a left-sided CDH. One patient was a diamniotic-dichorionic twin and underwent in utero selective reduction. Eighteen patients survived until birth. Eight patients survived to surgical repair, and all subsequently survived until discharge. Ten patients died after delivery. Four patients died before transfer to the Neonatal Intensive Care Unit. One patient was placed on extracorporeal membranous oxygenation (ECMO), which was complicated by a severe intracranial bleed. ECMO was stopped and the patient’s parents elected to withdraw care. Five patients were placed on palliative care after the family decided against or the patient was not found to be a candidate for ECMO. Of the five patients that were palliated, four had an initial pCO2 level greater than 80. One of these four patients, one had hypoplastic left heart syndrome, two had dextrocardia, the other patient had severe IUGR with a birth weight of 1800g at 34 weeks gestation (birth weight percentile of 8%). The remaining patient had an initial pCO2 of 66.1- however; the patient failed conventional ventilation, and was transitioned to HFOV with worsening acidosis. This patient had a birthweight of 2010 grams and was considered too small for ECMO, thus palliation was elected due to lack of further interventions.
Fetal and neonatal characteristics of the 19 left-sided CDH patients were recorded (Table 1). Maternal and/or neonatal records were reviewed to obtain information regarding gestational age at diagnosis, imaging measurements of prognosis, birth characteristics (gestational age and weight). We also recorded the type of ventilation required (i.e. if high-frequency oscillation was required), and the need for inhaled nitric oxide. Charts were reviewed for initiation of ECMO, or if ECMO had been offered. If available, any information regarding associated anomalies was recorded. Finally, if the fetus survived to surgical repair, the type of repair and need for a patch was noted.
Table 1. Fetal and Neonatal characteristics. U/S LHR = ultrasound lung-to-head ratio. U/S O/E LHR = ultrasound observed-to-expected lung-to-head ratio. MRI O/E FLV = MRI observed-to-expected fetal lung volume. HFOV = High frequency oscillatory ventilation. iNO = inhaled nitric oxide. ECMO = extracorporeal membrane oxygenation. ASD = atrial septal defect.| Characteristic | Value (n=18) |
|---|---|
| Gestational age at diagnosis (weeks) | 22.9 ±1.3 |
| U/SLHR | 1.4 ± 0.2 |
| U/S O/E LHR | 33.4 ± 4.1% |
| MRI O/E FLV | 32.1 ± 0.7% |
| Gestational age at birth for live deliveries (weeks) | 37.4 ± 0.6 |
| Birth weight (g) | 2908 ± 158 |
| HFOV | 10 (62.5%) |
| iNO | 10 (62.5%) |
| ECMO | 3 (20.0%) |
| Surgery | |
| Thoracoscopic | 1 (12.5%) |
| Open | 7 (87.5%) |
| Patch | 4 (50.0%) |
| Associated Anomalies | 3 (25.0%) |
| Cardiac | 3 (25.0%) |
| ASD | 2 (16.7%) |
| Hypoplastic Left Heart Syndrome | 1 (8.3%) |
Accuracy of U/S LHR
Initially, 10 patients were identified with prenatally diagnosed CDH who had undergone both prenatal ultrasound and MRI. The ultrasounds for these 10 patients were reviewed by a single maternal fetal medicine doctor (EP) for accuracy of the U/S LHR measurement. These patients underwent a total of 26 ultrasounds between September 2012 and February 2015. Upon reviewing these ultrasounds, 7 (26.9%) U/S LHR measurements were noted to be inaccurate. Five (71.4%) of the inaccurate measurements were due to the U/S LHR being calculated at a plane that did not include the four-chamber view of the heart.
After feedback was given to the ultrasonographers regarding the inaccuracies of the measurements and the most common error, ultrasounds from 11 more patients who had the prenatal diagnosis of CDH and had undergone both prenatal ultrasounds and MRIs were again evaluated for the accuracy of U/S LHR by the same maternal fetal medicine doctor (EP). These 11 patients underwent 33 ultrasounds. Three (9.1%) of these ultrasounds had inaccurate U/S LHR measurements. Not only did the number of inaccurate measurements decrease dramatically, but none of these inaccuracies were due to the plane of the measurement. Each of these inaccuracies was due to an oblique angle where the lung area was identified.
Predictors of Outcome
U/S LHR, U/S O/E LHR, and MRI O/E FLV measurements were compared between survivors and non-survivors. There was no statistical difference in the U/S LHR, or U/S O/E LHR for survivors versus non-survivors (Table 2). In contrast, the MRI O/E FLV was statistically different between the two groups.
Table 2. Imaging derived prognostic indicators for CDH stratified by survivors and non-survivors.| Survivors | Non-survivors | P-value | |
| U/S LHR | 1.8 ± 0.4 | 1.1 ± 0.1 | 0.08 |
| U/S O/E LHR | 42.2 ± 8.3% | 27.0 ± 2.9% | 0.07 |
| MRI O/E FLV | 45.8 ± 7.5% | 18.3 ± 2.6% | 0.001 |
When patients were divided based on the position of their stomach on MRI, there were statistically significant differences in U/S LHR, U/S O/E LHR, and MRI O/E FLV (Table 3). In all cases, the patients with no or some stomach in the thorax had significantly higher values than those with all of their stomach in their chest.
Table 3. Correlation of stomach position with imaging study| No/Some Stomach | All Stomach | P-value | |
| U/S LHR | 2.2 ± 0.7 | 1.1 ± 0.1 | 0.03 |
| U/S O/E LHR | 50.7 ± 14.4 | 28.8 ± 3.0 | 0.03 |
| MRI O/E FLV | 53.8 ± 6.8% | 24.2 ± 4.6% | 0.006 |
Eleven patients had an U/S LHR ≤ 1, and 8 (72.7%) of those patients died. Six patients had an U/S LHR >1.4, and only 4 survived (66.7%). Both of the patients died prior to transfer to the neonatal intensive care unit. One patient died at an outside hospital prior to transfer. The other patient died in the delivery room, after requiring chest compressions for bradycardia. The initial radiographs showed portal venous gas, and no aerated lungs with initial pCO2 >180. Nine of our patients had an U/S O/E LHR < 25% ,and 7 (77.8%) of those patients died. Four patients had an U/S O/E LHR ≥46%, and 1 (25%) of those patients died. This was the same patient with an U/S LHR > 1.4 who died prior to transfer from an outside hospital. Eleven patients in our cohort had MRI O/E FLV ≤25%, and 2 (18.2%) survived. Four patients had MRI O/E FLV ≥46%, of which all survived.
If one simply looks at the fetal data and predicts that those patients with an U/S LHR < 1, U/S O/E LHR < 15%, or MRI O/E FLV <25% are expected to die, the U/S LHR correlated with outcome for 13 (68.4%) patients. Likewise, U/S O/E LHR correlated with outcome for 13 (68.4%) patients. The U/S LHR and U/S O/E LHR correlated for 17 (89.4%) patients. The MRI O/E FLV correlated with outcome in 13 (68.4%) patients.
Of the 19 patients, 4 had none or some stomach in the thorax on MRI. All 4 of these patients survived until discharge. Fifteen patients had all their stomach in the thorax on MRI. These patients had significantly lower U/S LHR, U/S O/E LHR ,and MRI O/E FLV (Table 3). Only 21% of these patients survived.
Discussion
Starting a fetal program requires the ability to offer appropriate prenatal counseling. Pediatric surgeons, neonatologists, and maternal fetal medicine providers should be involved in these discussions, especially in CDH patients. Furthermore, in order to offer appropriate counseling for CDH, the program must also be able to provide adequate imaging, and accurate interpretation of that imaging, to providefor better prognostication.
Prognostic indicators utilized in left-sided CDH do not necessarily correlate with outcomes for right-sided CDH. Research has shown that right-sided and bilateral CDH may have different prognoses17, 18. Therefore, it is it important to counsel those patients differently. These patients were excluded from our analysis.
While there is no clear consensus on which imaging modality or measurement to use for prognostication in left-sided CDH, U/S LHR is the most common modality used. This measurement is calculated in one of three ways. The three methods differ in how the area of the right lung (for left-sided CDH) is calculated. For all three methods, the right lung is identified at the level of the four-chamber view of the heart. Likewise, for all methods, the area of the right lung is divided by head circumference. One method is to estimate the right lung area (in left-sided CDH) by measuring the longest diameter of the lung and multiplying that by the longest perpendicular value. The second method also involves measuring the cross-sectional area of the right lung by was obtained by multiplication of the anteroposterior diameter of the lung at the mid-clavicular line by the perpendicular diameter at the midpoint of the anteroposterior diameter. For the third method, the border of the right lung is traced15. Each method has its limitation in reproducibility and reliability; however, as mentioned above, all require the measurements to be taken at the level of the 4-chamber view of the heart. We have shown in this quality improvement project that lack of the 4-chamber view of the heart was the most common error ultrasonographers made when learning to obtain the U/S LHR. It is important for both ultrasonographers, and fetal providers to be aware of this pitfall. In our second round of ultrasounds after the quality improvement feedback was given, the error abated. LikewiseAdditionally, fetal providers can quickly evaluate the ultrasound images for this error, prior to prenatal counseling, and use this information to determine the validity ofhow heavily to rely on the U/S LHR for prognostication.
An U/S LHR < 1 is a predictor of poor prognosis. Studies have suggested a 75-100% mortality rate with an U/S LHR < 119, 20. In our current study, 11 patients had an U/S LHR ≤ 1, and 8 (72.7%) of those patients died. On the other hand, an U/S LHR > 1.4 is predictive of 85-100% survival19, 20, 21. In this study, 6 patients had an U/S LHR >1.4, and only 4 survived (66.7%) in this group. Our U/S LHR data was less accurate at predicting survivors and non-survivors than has been typically referenced, even when only the validated U/S LHR values were utilized.
In addition to being operator-dependent, another limitation of U/S LHR is its reliance on gestational age. It has been shown that there is an 18-fold increase in lung area between 12 and 32 weeks of gestation14. In contrast, there is only a 4-fold increase in head circumference14. In an attempt to overcome this dependence on gestational age, many have utilized the U/S O/E LHR, which compares the measured U/S LHR to a normogram of U/S LHR for healthy fetuses at the same gestational age. Alfaraj et. al have demonstrated an approximate 75% mortality rate of approximately 75% with an U/S O/E LHR ≤25%, and 100% survival with an U/S O/E LHR ≥46%22. Nine of our patients had an U/S O/E LHR < 25%, and 7 (77.8%) of those patients died, similar consistent with theto published data. Four patients had an U/S O/E LHR ≥46%, and only 3 (75%) of those patients survived until discharge. We areAlthough we are limited by small patient size in this study group, but our data highlights the need for a better prognostic measurements especially in light of the fact thatsince both of these ultrasound measurements are user dependent with a steep learning curve16. Additionally, these values are important as they are often are utilized when for consideration ofing fetoscopic tracheal occlusion evaluation for in patients with severe CDH.
MRIs are an additional imaging modality often used in the work up of CDH for prenatal counseling and better prognostication. The MRI can be used to measure fetal lung volumes, which are compared to normograms from healthy fetuses, to obtain the MRI O/E FLV based on gestational age. Previously published data suggest that MRI O/E FLV ≤25% is associated with a 100% mortality, and MRI O/E FLV ≥46% is associated with a nearly 90% survival rate22. Eleven patients in our cohort had MRI O/E FLV ≤25%, and 9 (81.8%) of them died. Four patients had MRI O/E FLV ≥46%, and all survived. While this measurement was better at predicting survival in our cohort, it still did not accurately predict mortality. Furthermore, as mentioned in the introduction, obtaining the MRI FLV requires special software, and a highly trained radiologists. to obtain the measurements. It is also time-consuming to obtain as each individual slice of the MRI must be analyzed and the lungs traced. Thus, MRI O/E FLV is also is not always feasible, and may not be an ideal prognostic indicator for CDH.
Since none of these typical prognosticators are ideal and other measurements such as the McGoon index are also complex to obtain, we investigated simply using stomach position on MRI as a prognostic indicator. The location of the stomach serves as a marker of defect size, visceral herniation, and pulmonary hypoplasia. Here we report that those patients with some or no stomach in the thorax have significantly higher U/S LHR, U/S O/E LHR, and MRI O/E FLV than those with all of their stomach in the chest. Furthermore, all patients who had just some or no stomach in the chest on MRI survived to surgery and discharge. Therefore, lack of the entire stomach in the thorax on MRI is a good prognostic indicator.
The stomach position on MRI matched the expected outcome in 15/ out of 19 (78.9%) patients, while U/S LHR, U/S O/E LHR or MRI O/E FLV matched the expected outcomes in 13/19 (68.4%) patients. These 13 patients were not the same patients in all groups. While there was not a statistical difference between the ability of stomach position on MRI to predict outcome compared to U/S LHR, U/S O/E LHR, or MRI O/E FLV (p=0.71 for all comparisons), stomach position on MRI is not inferior as a prognostic indicator in CDH for this cohort of patients. Stomach position on MRI also has the advantage that it is quick and easy to obtain by a radiologist without specific fetal expertise. We propose that this finding be utilized in addition to, or as an alternative of for more complex and less reproducible measures.
This study is limited by being a single center study with a small sample size. Likewise Additionally, nearly half (5/11) of the patients who did not survive to discharge underwent were palliatedion. Therefore us, it is impossible to know if they would have survived to discharge if heroic measures had been performed. The presence of additional undiagnosed structural and chromosomal abnormalities also may have contributed to non-survival in some patients. Yet, given However, given the high levels of pCO2 on their initial blood gas, their known associated associated anomalies, and/or the trajectory of their clinical course, it is unlikely that most would have survived. Therefore, we elected to include these patients were included in the study analysis. Despite the aforementioned limitations, the data is compelling. Further prospective trials will be required to further validate this data.
In summary, the most common error when sonographers are initially learning to obtain LHR measurements, the most common error made by ultrasonographers during LHR meausurement is obtaining the measurement at a level other than at the four-chamber view of the heart. Fortunately, for both ultrasonographer and fetal providers reviewing the ultrasounds, this is easy to identify and correct. Simply identifying this common problem reduced erroneous measurements dramatically. Unfortunately, even after improving the accuracy of the LHR measurement, both U/S LHR and U/S O/E LHR were not as reliable as typically reported for prognosis. MRI FLV had improved prognostic value in our study, but it is time consuming and requires a specially trained radiologist. Our data suggest that stomach position on MRI has similar prognostic value to U/S LHR and MRI FLV, and furthermore, is reproducible, fast, and easy to obtain.
References
- 1.Badillo A, Gingalewski C. (2014) Congenital diaphragmatic hernia: Treatment and outcomes.SeminPerinatol. 38(2), 92-96.
- 2.Losty P D. (2014) Congenital diaphragmatic hernia: Where and what is the evidence?SeminPediatrSurg. 23(5), 278-282.
- 3.Skari H, Bjornland K, Haugen G, Egeland T, Emblem R. (2000) Congenital diaphragmatic hernia: A meta-analysis of mortality factors.JPediatrSurg. 35(8), 1187-1197.
- 4.Bojanic K, Woodbury J M, Cavalcante A N. (2017) Congenital diaphragmatic hernia: Outcomes of neonates treated at mayo clinic with and without extracorporeal membrane oxygenation.PaediatrAnaesth. 27(3), 314-321.
- 5.Stoffan A P, Wilson J M, Jennings R W, Wilkins-Haug L E, Buchmiller T L. (2012) Does the ex utero intrapartum treatment to extracorporeal membrane oxygenation procedure change outcomes for high-risk patients with congenital diaphragmatic hernia?JPediatrSurg. 47(6), 1053-1057.
- 6. (1997) Inhaled nitric oxide and hypoxic respiratory failure in infants with congenital diaphragmatic hernia. the neonatal inhaled nitric oxide study group (NINOS).Pediatrics. 99(6), 838-845.
- 7.Barrington K J, Finer N, Pennaforte T, Altit G. (2017) Nitric oxide for respiratory failure in infants born at or near term.Cochrane Database Syst Rev. 1-000399.
- 8.Mugford M, Elbourne D, Field D. (2008) Extracorporeal membrane oxygenation for severe respiratory failure in newborn infants.Cochrane Database Syst Rev.(3):CD001340.doi(3):CD001340.
- 9. (1996) UK collaborative randomised trial of neonatal extracorporeal membrane oxygenation. UK collaborative ECMO trail group.Lancet. 348(9020), 75-82.
- 10.Skari H, Bjornland K, Frenckner B. (2004) Congenital diaphragmatic hernia: A survey of practice in scandinavia.PediatrSurg Int. 20(5), 309-313.
- 11.Javid P J, Jaksic T, Skarsgard E D, Lee S. (2004) Canadian Neonatal Network. Survival rate in congenital diaphragmatic hernia: The experience of the canadian neonatal network.JPediatrSurg. 39(5), 657-660.
- 12.Steinhorn R H, Kriesmer P J, Green T P, McKay C J, Payne N R. (1994) Congenital diaphragmatic hernia in minnesota. impact of antenatal diagnosis on survival.ArchPediatrAdolescMed. 148(6), 626-631.
- 13.Samangaya R A, Choudhri S, Murphy F, Zaidi T, Gillham J C et al. (2012) Outcomes of congenital diaphragmatic hernia: A 12-year experience.PrenatDiagn. 32(6), 523-529.
- 14.Jani J C, Peralta C F, Nicolaides K H. (2012) Lung-to-head ratio: A need to unify the technique.UltrasoundObstetGynecol. 39(1), 2-6.
- 15.Victoria T, Danzer E, Adzick N S. (2013) Use of ultrasound and MRI for evaluation of lung volumes in fetuses with isolated left congenital diaphragmatic hernia.SeminPediatrSurg. 22(1), 30-36.
- 16.Cruz-Martinez R, Figueras F, Moreno-Alvarez O. (2010) Learning curve for lung area to head circumference ratio measurement in fetuses with congenital diaphragmatic hernia.UltrasoundObstetGynecol. 36(1), 32-36.
- 17.Botden S M, Heiwegen K, van Rooij IA. (2016) Bilateral congenital diaphragmatic hernia: Prognostic evaluation of a large international cohort.JPediatrSurg.
- 18.Victoria T, Danzer E, Oliver E R. (2017) Right congenital diaphragmatic hernias: Is there a correlation between prenatal lung volume and postnatal survival, as in isolated left diaphragmatic hernias?FetalDiagnTher.
- 19.Laudy J A, M Van Gucht, Van Dooren MF, Wladimiroff J W, Tibboel D. (2003) Congenital diaphragmatic hernia: An evaluation of the prognostic value of the lung-to-head ratio and other prenatal parameters.PrenatDiagn. 23(8), 634-639.
- 20.Yoshimura S, Masuzaki H, Hiraki K, Miura K, Nakayama D et al. (2005) Congenital diaphragmatic hernia: An evaluation of the prognostic value of the lung-to-head ratio.J MedUltrason(2001). 32(3), 115-119.
